Process Flow Diagram for Urea Production with Full Liquid Recycling
Scene 1 (0s)
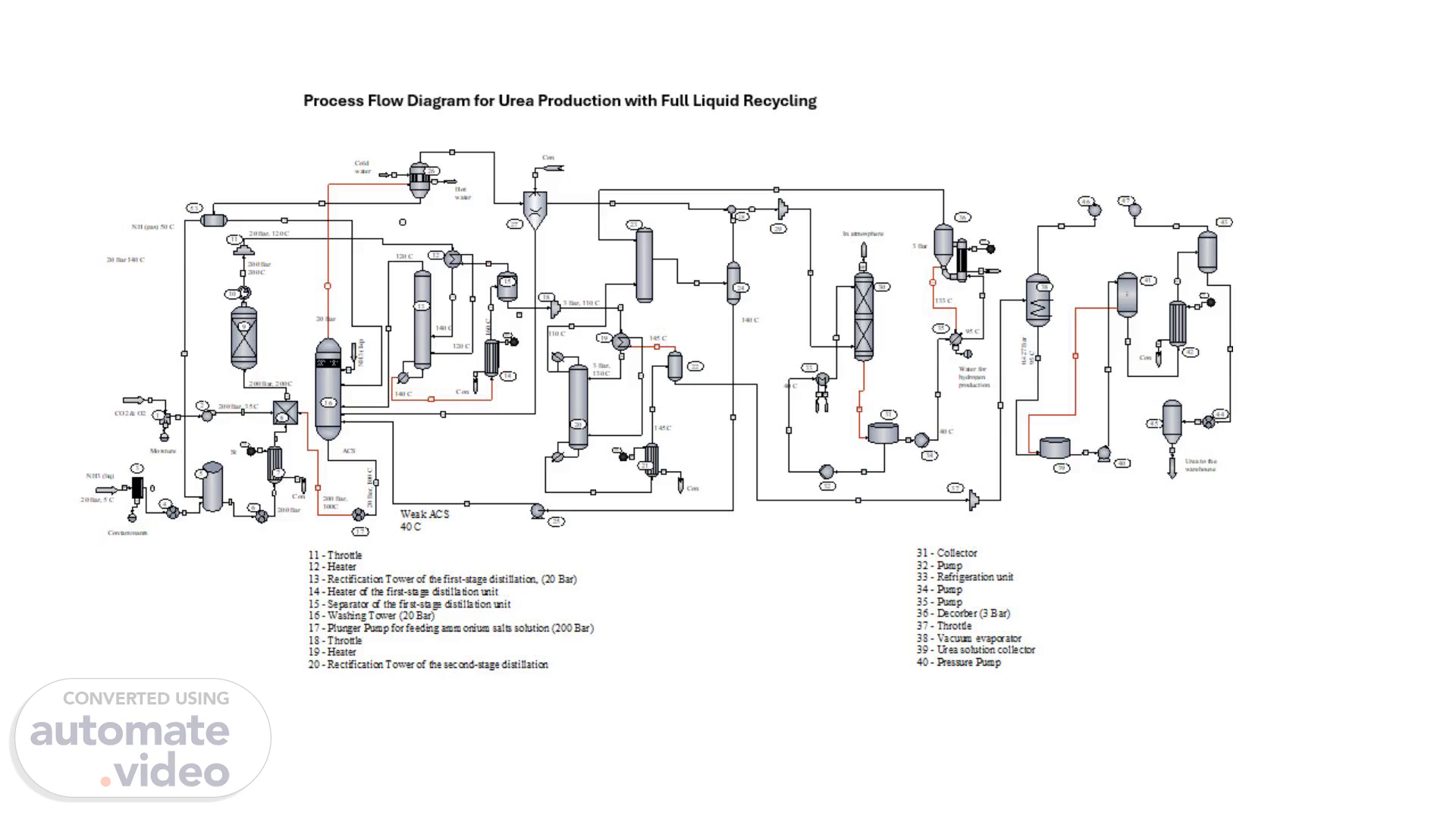
[Audio] Process Flow Diagram for Urea Production with Full Liquid Recycling Carbon dioxide from the gas tank having previously passed through a Moisture Separator (1) enters Compressor (2) which supplies it to Mixer (8) at a pressure of 200 bar and a temperature of 35 degrees celsius. To prevent corrosion of the apparatus carbon dioxide is mixed with oxygen in an amount of 0.6-1 volume %; the oxygen contributes to the creation of a stable passivating film. Liquid ammonia from the warehouse under pressure 20-25 bar passes through a Filter (3) to remove oil and other contaminants and is supplied by Pump (4) to the liquid ammonia Collector (5) which also receives return liquid ammonia from Condenser (21) through Receiver (22). From Collector (5) liquid ammonia is pumped by Plunger Pump (6) under a pressure of 200 bar and is sent through Heater (7) to Mixer (8). In the heater liquid ammonia is heated with steam to a temperature that ensures autothermal synthesis occurs. Mixer (8) simultaneously receives carbon dioxide and ammonia not converted into urea in the form of a solution of ammonium salts. Thus liquid ammonia carbon dioxide and firewood are supplied to the mixer in a molar ratio of 4.5:1:0.5. The mixer is designed to thoroughly mix all three components resulting in the formation of ammonium carbamate. The process in the mixer takes place at 180-200 degrees celsius and 200 bar. From the mixer the melt enters the Synthesis Column (9) in which urea is formed at 200 degrees celsius and 200 bar. The residence time (10) of the reaction mixture in the synthesis column is 45 minutes. Under these conditions the degree of conversion of ammonium carbamate to urea is 65%. The urea melt formed in the synthesis column is throttled from 200 bar to 20 bar and sent to the first-stage distillation unit. When the pressure of the melt decreases in the Throttle (11) from 200 bar to 20 bar its temperature drops to 120 degrees celsius. The distillation unit consists of a Heater (12) a Rectification Tower (13) a Heater (14) and a Separator (15). Before the melt enters the Rectification Tower of the first-stage distillation it is heated to 140 degrees celsius by gases from the Separator (15) which are used to evaporate part of the ammonia from the melt. The gases from the Separator (15) are cooled to 120 degrees celsius after transferring their heat to the melt. The liquid phase from the distillation column goes to Heater (14) in which the steam is heated to 160 degrees celsius. From the heater the vapor-liquid mixture enters Separator (15) where the gas and liquid phases are separated. The liquid phase from Separator (15) is sent to a similar second-stage distillation unit and the gaseous phase flows down the Rectification Tower (13). In the rectification tower the gas phase is mixed with gaseous ammonia released from the melt during throttling and sent to Washing Tower (16). The washing tower is irrigated with liquid ammonia which supplied to the washing column for temperature regulation and a weak solution of ammonium salts coming from the separator (24) into which the solution of ammonium salts comes from the second-stage condenser (23). Pure gaseous ammonia from the washing column at a temperature of 45-50 degrees celsius enters the First-Stage Condenser (26) where it is condensed and discharged into the Ammonia Collector (5) for return to the cycle. The liquid phase from the washing column which is a solution of ammonium salts concentrated due to the absorption of C-O-2 and N-H-3 with a.
Scene 2 (4m 39s)
[image] 02 203=5 c ZOOC 2m Bar 110 c 140 C weak ACS 40 c.
Scene 3 (5m 45s)
Pure gaseous ammonia from the washing column at a temperature of 50°C enters the First-Stage Condenser (26), where it is condensed and discharged into the Ammonia Collector (5) for return to the cycle. The liquid phase from the washing column, which is a solution of ammonium salts concentrated due to the absorption of CO2 and NH3 with a temperature of 105°C, enters Plunger Pump (17) and is supplied to the mixer under a pressure of 200 bar. Gases with a small ammonia content that are not condensed in the condensers of the first and second stages are sent to Absorber (30). The liquid phase after Separator (15) is throttled to a pressure of 3 bar and added to the second-stage distillation unit, consisting of a Heater (19) a Rectification Tower (20), a Heater (21), and a Separator (22). When throttling, the temperature of the liquid phase decreases to 110°C. Before, the liquid phase enters Rectification Tower (20), it is heated in the Heater (19) to 130°C by gases coming from Separator (22) at a temperature of 145°C. From the Tower (20) liquid phase feeding the heater (21), in which it heated by steam to 145°C. In the Heater (21) ammonium carbamate is finally decomposed, and excess ammonia is distilled off from the solution. From Heater (21), the vapor-liquid mixture enters Separator (22), where the liquid phase is separated from the gaseous one. The liquid phase is a 72% urea solution and is sent for processing into the finished product. The gaseous phase returns to the bottom of the distillation column, where it, having given up part of the heat to heat the solution from 110 to 130°C, goes to the second-stage Condenser (23). Here, water vapor is condensed due to the absorption of CO2 and NH3, and the condensate forms a weak solution of ammonium salts, which is returned by Pump (25) for concentration into Washing Column (16) and then into Mixer (8)..
Scene 4 (6m 53s)
Gases are purified from ammonia before being released into the atmosphere using a system consisting of an Absorber (30) and a Desorber (36). Unabsorbed gases with small admixtures of ammonia from the condensers of the first and second stages enter Absorber (30), which is irrigated with steam. The weak solution of ammonia and ammonium salts formed in the absorber is then sent to Desorber (36), where ammonia is separated from the solution and returned to the second-stage condenser. Water from the Desorber, containing small impurities of ammonium carbonate, is sent to reverse osmosis for purification. The purified water is then used in hydrogen production. Finally, gases purified from ammonia are released from the absorber into the atmosphere. The liquid phase from the Second-stage Separator (22) enters the Vacuum Evaporator (38), where due to self-evaporation it condenses to 76%. Then, in film-type Evaporators (42) and (43), the solution is evaporated to 98.5%. The melt is then sent to the Granulation tower (45) and subsequently to packaging..