PowerPoint Presentation
Scene 1 (0s)
[Audio] Hi everyone and welcome to this presentation on nitrosamine impurities. Today, we will be taking a look at what they are, how they are formed, and the potential risks they can pose to drug products. So, let's start right away!.
Scene 2 (16s)
[Audio] NITROSAMINE is a chemical compound found in some materials and products, with potential adverse effects on human health. To reduce these risks, AI limits are set to regulate the maximum amount of NITROSAMINE that can be present in a product or material. In this presentation, I will cover the introduction of NITROSAMINE and the AI limits associated with it..
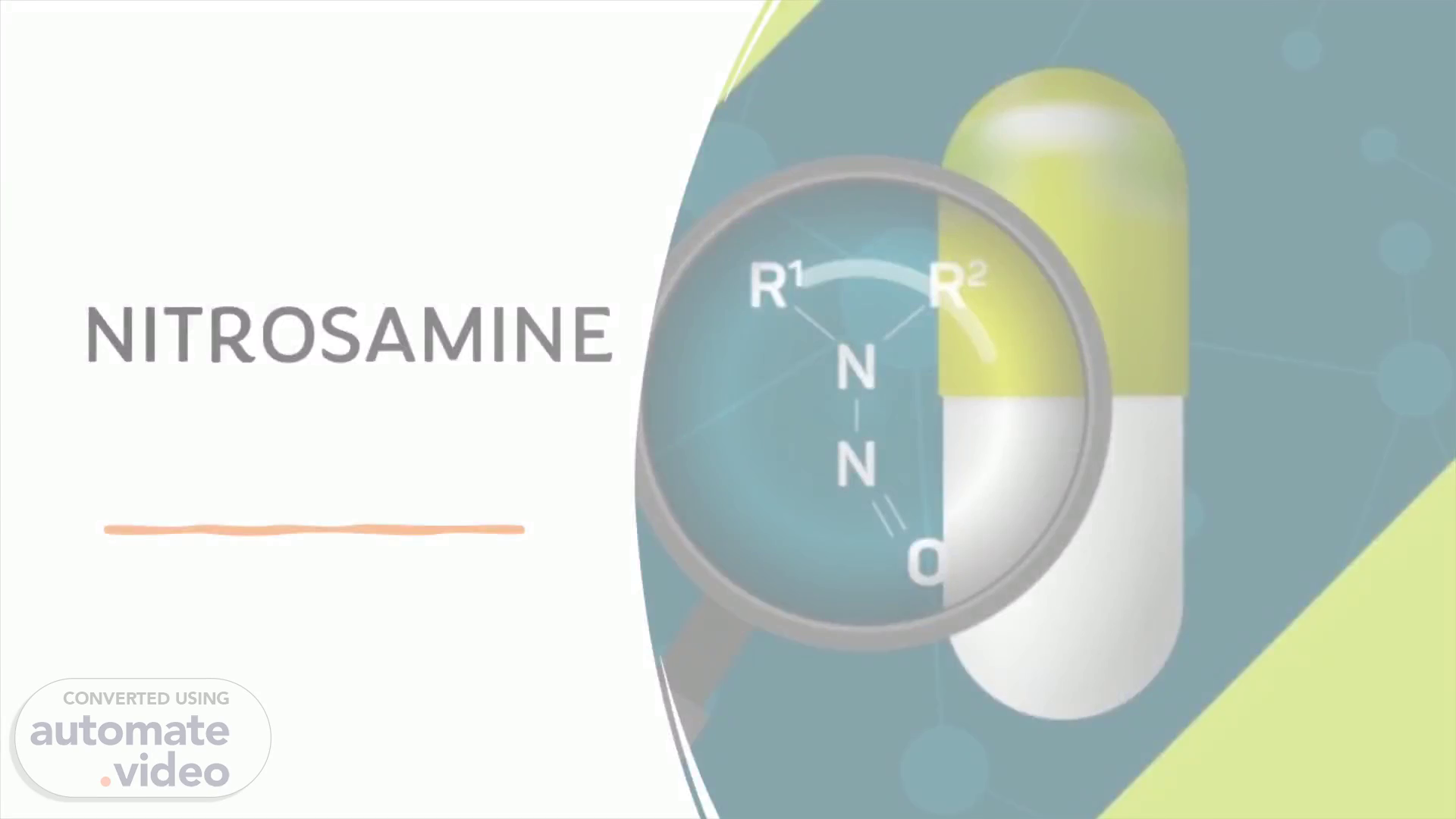
Scene 3 (40s)
[Audio] Nitrosamine impurities are organic compounds formed when nitrogen-containing chemicals, such as nitrites and amines, react with certain substances. There is a potential risk to public health due to the possibility of these compounds being present in various medications. Reports have associated nitrosamine impurities with cancer, liver, and kidney damage. It is, therefore, important to pay attention and regulate these compounds in order to protect the safety of the public. To do this, it is essential to comprehend what nitrosamine impurities are and how they are formed..
Scene 4 (1m 18s)
[Audio] Nitrosamines are chemicals that can be found in the environment and in some drugs, with the presence of nitrosamine impurities, known as N-nitrosodimethylamine (NDMA), detected in the class of blood pressure medications known as sartans, having sparked global concern since 2018. This has developed into a major issue for regulators worldwide, as the presence of nitrosamines in drug products remains a major source of worry..
Scene 5 (1m 45s)
[Audio] When looking at certain medicines, it is important to consider the risk associated with N-nitrosamine impurities. The formation of such impurities can occur when a nitrosating agent is combined with a secondary or tertiary amine and the right conditions. Thus, it is essential to assess the ingredients of the medicine during the risk assessment process..
Scene 6 (2m 8s)
[Audio] Nitrosamine Impurities are a family of chemicals that may be linked to cancer. These chemicals are generated when secondary amines, amides, carbamates or urea-derivatives come into contact with nitrite or other nitrogenous agents. Of note, N-nitrosamines have been recognized as possible mutagenic carcinogens. Knowing about these compounds and how they are formed is an important factor in safeguarding against health issues..
Scene 7 (2m 37s)
[Audio] Now let's take a look at how nitrosamines are formed in drug products. Nitrite can form the highly reactive species nitrous anhydride (N2O3) under mildly acidic conditions. This nitrite can then react with secondary or tertiary amines present in excipients to form N-nitrosamines. Additionally, the presence of nitrates in excipients can react with enzymes to form nitrite, which can also form N-nitrosamines. It is important to monitor for these impurities to ensure drug safety..
Scene 8 (3m 13s)
A magnifying glass over pills Description automatically generated.
Scene 9 (3m 30s)
[Audio] Nitrosamines are chemical compounds that can be formed under highly specific conditions. Nitrite salts, secondary, tertiary, or quaternary amines, and acidic reaction conditions are all ingredients for the formation of nitrosamines. Nitrite salts form nitrous acid which reacts with an amine to form the final nitrosamine product. It's important to be aware of these conditions and know how to prevent them from occurring in order to avoid the formation of these potentially hazardous compounds..
Scene 10 (4m 3s)
[Audio] Observing the slide, it covers nitrosamine impurities mentioning three specific ones: dimethylformamide, dimethylamine, and N-nitrosodimethylamine. It is understood that these nitrosamines could be generated in the manufacturing process due to the presence of secondary, tertiary, and quaternary amines. This is a notable factor to take into consideration when evaluating the quality of a drug or other product..
Scene 11 (4m 32s)
[Audio] A common issue in the pharmaceutical industry is the contamination of vendor-sourced materials, such as starting materials and raw materials. In particular, nitrosamine impurities can be introduced when these materials are contaminated. The FDA has observed potentially dangerous cases of this type of contamination, for example when fresh solvent is shipped from vendors or when nitrate containing raw materials, such as potassium nitrate, contain nitrite impurities. It is important that vendors take necessary steps to ensure the quality and safety of their materials..
Scene 12 (5m 8s)
[Audio] Nitrosamines are chemical compounds that pose health risks in certain concentrations. They can be present in recovered solvents, reagents, and catalysts, which can contaminate the end-product if not properly purified. Nitrous acid used in the recovery process to decompose residual azide can cause the formation of nitrosamines. If their boiling point or solubility is similar to the recovered materials, they can become entrained and present in the recovered materials. Companies must follow strict purification practices during recovery to avoid potential risks to human health..
Scene 13 (5m 47s)
[Audio] Nitrosamine impurities can be formed when nitrous acid is added directly to the reaction mixture during the quenching process. This can happen if the raw materials used in the manufacturing process have residual amines. Quenching this way raises the risk of nitrosamine formation, therefore it should be prevented whenever possible..
Scene 14 (6m 9s)
[Audio] Nitrosamines are dangerous chemicals that can form during the manufacture of active pharmaceutical ingredients (APIs). One possible cause of the presence of nitrosamine impurities is the failure to optimize the manufacturing process. The FDA has noted cases where there was little to no control of conditions such as temperature, pH, or the introduction of reagents, intermediates, or solvents. To ensure an API of high quality and safety, it is vital that these variables are managed correctly..
Scene 15 (6m 43s)
[Audio] We are going to discuss nitrosamine impurities and their acceptable limits. Nitrosamines are organic compounds that form when certain nitrogen-containing compounds react with nitrous acid or its salts. These compounds can be found in food, beverages, pharmaceuticals, and other products. It is essential to understand the acceptable limits of nitrosamine impurities in these products, to guarantee their safety for human use..
Scene 16 (7m 12s)
[Audio] Nitrosamine compounds are formed from the reaction of proteins and nitrites, a type of preservative commonly used in food products. The FDA has established Acceptable Intake (AI) limits for six nitrosamine impurities – NDMA, NDEA, NMBA, NMPA, NIPEA, and NDIPA. To protect human health, the Recommended AI limits are below these established limits, as seen in the table. This Recommended AI limit serves as the basis of understanding on nitrosamine impurities..
Scene 17 (7m 50s)
[Audio] We will now discuss the part of the capsule manufacturing process that involves close-up examination of a blue liquid in order to detect impurities from nitrosamines. This method should be used by manufacturers to detect any impurities before releasing the capsule..
Scene 18 (8m 7s)
[Audio] Discussions about a frequently disregarded aspect of capsule production are necessary: the possible presence of nitrosamine impurities. Negligence in this area could result in critical health implications. To try and avert the emergence of nitrosamine impurities, capsules are mostly made up of gelatin and HPMC, having various amino acids. Additionally, procedures such as strong acidic conditions, solvents and nitrosating agents which might contribute to the formation of nitrosamine impurities must be avoided..
Scene 19 (8m 42s)
[Audio] Our product, “Hard Gelatin Capsule Shells & Cellulose Capsule Shells”, is free of Nitrosamine impurity, so our customers can be assured that there is no risk of any presence of Nitrosamine impurities in our products. Slide number 19 of our presentation is about Nitrosamine impurities. Thank you for your attention..