Scene 1 (0s)
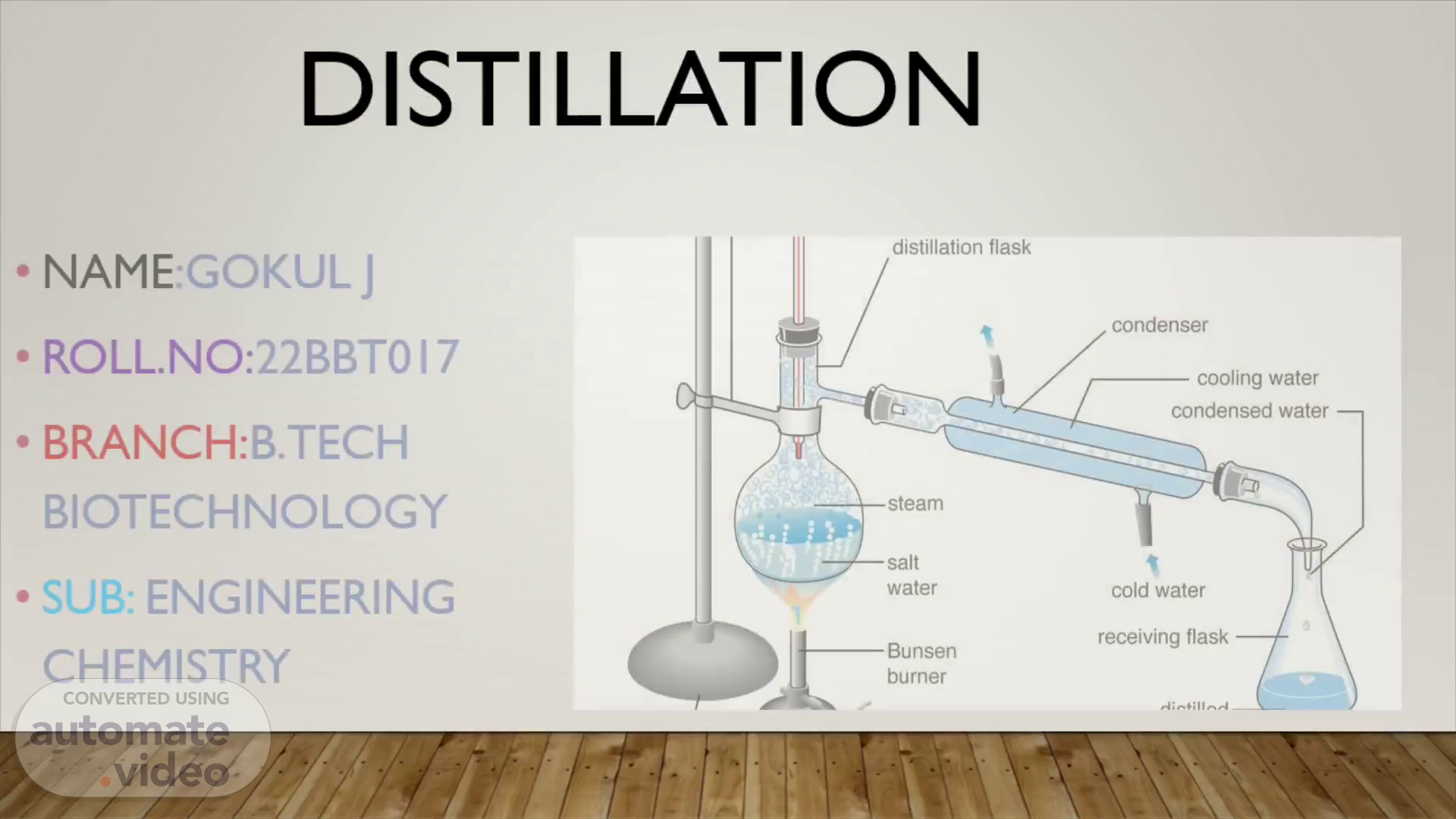
DISTILLATION. NAME :GOKUL J ROLL.NO: 22BBT017 BRANCH: B.TECH BIOTECHNOLOGY SUB: ENGINEERING CHEMISTRY.
Scene 2 (9s)
Definition Distillation is a process that involves separating and purifying liquids by heating them to create vapor and then cooling the vapor to produce concentrated liquid form..
Scene 3 (25s)
Types of distillation. FRACTIONAL DISTILLATION: Fractional distillation is a separation technique based on the differences in boiling points of the components in a liquid mixture. It uses a fractionating column to achieve separation by providing repeated vaporization and condensation cycles. The component with the lowest boiling point condenses and collects at the top, while higher boiling point components condense at lower levels within the column, resulting in the separation of the mixture. *Video in the next slide.
Scene 5 (53s)
TYPES OF distillation. STEAM DISTILLATION: Steam distillation separates volatile compounds from non-volatile substances effectively. it is gentle on heat-sensitive compounds, preserving their integrity during extraction. Steam distillation finds wide application in industries such as fragrance, flavor, herbal medicine, and chemical synthesis, offering a versatile method for obtaining pure and concentrated products. Here vapour pressure is extracted independently by an individual constituent on its own. The Vapour pressure of the system increases consequently, the two immiscible liquids start to boil when the vapour pressure of these liquids out place the atmospheric pressure. * Video in the next. Slide.
Scene 7 (1m 26s)
Applications of distillation. Distillation is used to remove impurities, contaminants, and minerals from water, making it safe for drinking and other purposes. It is essential in the production of alcoholic beverages, separating alcohol from the fermented mixture to increase its concentration. It is used in the refining of crude oil to separate it into different fractions, such as gasoline, diesel, and jet fuel, based on their boiling points. It is commonly employed to extract essential oils from plants, capturing the volatile aromatic compounds. It is widely used in the chemical industry for separating and purifying various chemicals, solvents, and intermediates used in the manufacturing of diverse products.
Scene 8 (1m 56s)
Factors affecting distillation. Temperature plays a crucial role in distillation as it determines the vaporization of the more volatile components, which can be collected and condensed separately. Pressure affects the boiling points of substances. By adjusting the pressure, it is possible to lower the boiling points of certain components, facilitating their separation during distillation. The presence of azeotropes (constant boiling mixtures) or close boiling point components can make separation challenging and may require additional techniques such as fractional distillation. The surface area available for vaporization and condensation affects the speed and efficiency of distillation. Increasing the surface area, often achieved through the use of trays or packing in the distillation column, enhances separation by allowing more interactions between the vapor and liquid phases. The rates at which the mixture is fed into the distillation column and the condensate is collected can impact the separation..
Scene 9 (2m 33s)
Challenges in distillation. Distillation processes often require significant amounts of energy to heat the feed mixture and vaporize the desired components. Finding ways to improve energy efficiency in distillation is a major challenge. Traditional distillation techniques can have a significant environmental impact due to the release of volatile organic compounds (VOCs) and greenhouse gases. Developing greener distillation methods that minimize emissions is a challenge. Azeotropic mixtures are challenging to separate because they have boiling points similar to their constituents, resulting in difficulty in achieving high purity. Overcoming the limitations of azeotropic distillation is a significant challenge in the field..
Scene 10 (2m 59s)
FUTURE TRENDS IN DISTILLATION. Energy Efficiency: Future trends in distillation will focus on improving energy efficiency by incorporating advanced technologies such as heat integration, waste heat recovery, and process intensification. This will help reduce the energy consumption and environmental impact of distillation processes. Advanced Materials: Distillation processes will benefit from the development and implementation of advanced materials, such as novel catalysts, membranes, and adsorbents. These materials will enable more selective separations, higher product purity, and improved process economics. Process Optimization and Automation: The future of distillation will involve increased process optimization and automation, leveraging advanced data analytics, machine learning, and artificial intelligence. This will enable real-time monitoring, control, and optimization of distillation operations, leading to improved process performance, reliability, and productivity..